Imagine, for a moment, our primitive planet. It’s over 4 billion years ago and Earth is swathed in carbon dioxide. The CO2 rich atmosphere gives way to carbonate rich water which, after evaporation, is left briny and alkaline. Carbon dioxide dissolved in the water allows a special chemical to escape from submerged rocks. This uncommon little chemical goes on to do something extraordinary — it proceeds to play an indispensable role in the emergence of life. Sighting of this chemical in the year 2020 leads scientists to believe, just for a moment, that there may be alien organisms on a nearby world. Yet as revolutionary as it is, the story of its origin remains uncertain.
The chemical element in question is phosphorus, abbreviated “P” in the periodic table. It is 1 of 6 elements — the others being carbon, hydrogen, nitrogen, oxygen and sulphur — on which our biology depends. Carbonates in those ancient lakes would have bonded with deposits of calcium, leaving early phosphorus molecules unattached and thus free to help kickstart the creation of life. Alkaline lakes are so rich in phosphorus that their rates of the element are 50,000 times higher than those of oceans and rivers. A then sterile Earth would’ve also meant there were no microbes around to consume and thus deplete these high phosphorus levels.
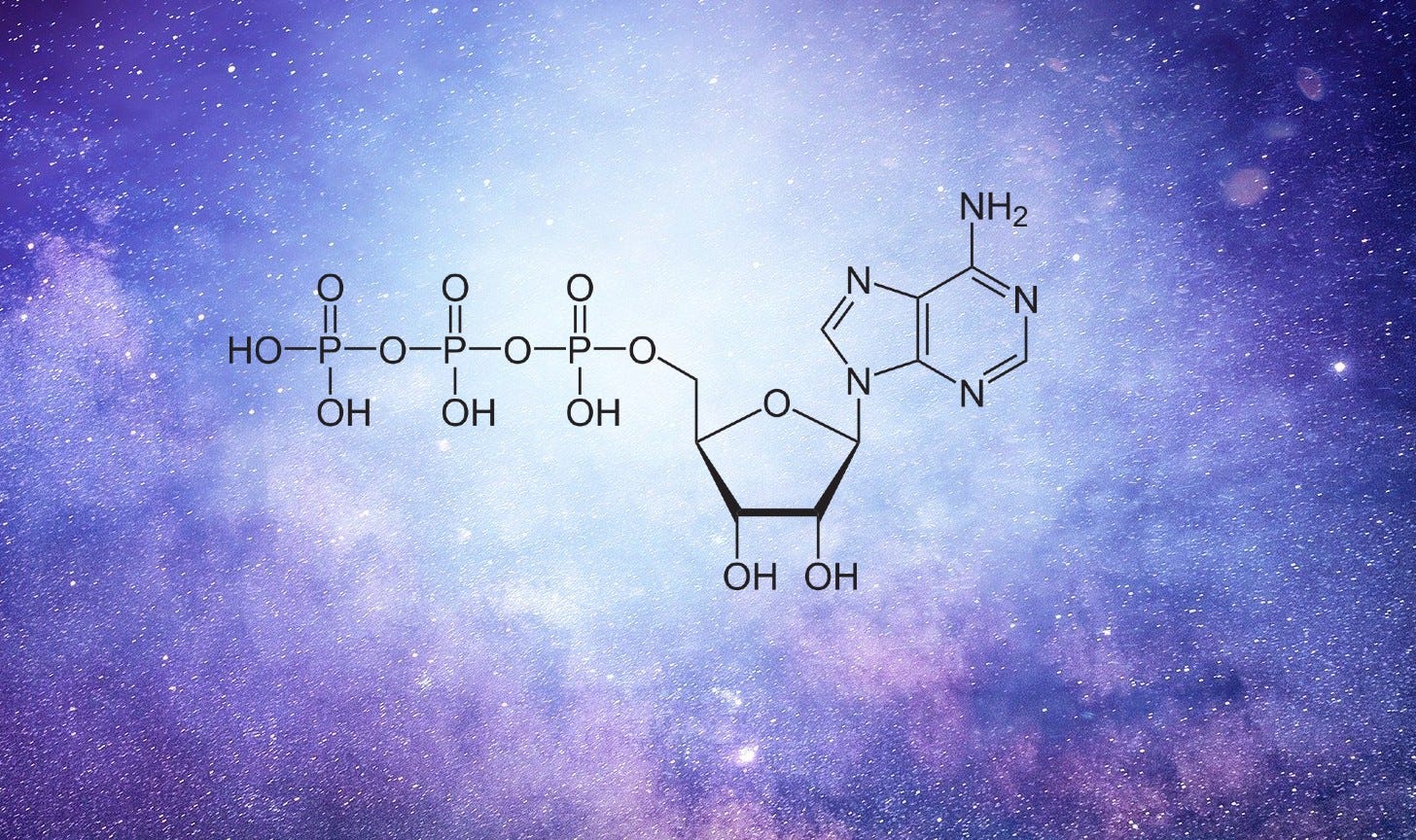
Cells within our bodies use phosphorus in their membranes but the element is also present in the very structure of our DNA and RNA. It’s an essential part of the energy-carrying molecule adenosine triphosphate (ATP) which helps store and transfer energy between cells. From biomolecules and fats, to phosphoproteins and teeth, phosphorus has integrated itself into the foundation of living organisms. To this day our bodies continue to acquire phosphorus from plants or via the consumption of plant-eating animals. It’s also an important ingredient in fertilizers. Our wasteful methods of farming are, however, quickly depleting the planet’s natural phosphorus reserves.
Yet despite how important phosphorus is to the creation and development of life on Earth, it’s the rarest of all 6 essential elements. It’s only the 17th most abundant element in the Solar System and is found in just .09% of the Earth’s crust. Overall a paltry .0007% of matter in the universe is phosphorus. The creation of this element has been traced back to the lifespan of stars, and especially to supernovae events which create phosphorous gas that becomes trapped within interstellar rocks. Rock pieces, ice, and other debris come together over the years to birth entire worlds — fresh new planets suffused with phosphorus in their crust. These planets can also receive added doses of the element in the form of comets and incoming meteorite collisions.
But preliminary research finds that we don’t yet have an accurate understanding of how much phosphorus a supernova event will create. Two separate nebulas — specifically the Cassiopeia A and the Crab nebula — have been found to have varying amounts of phosphorus in their widespread bodies of cosmic dust. This discrepancy may be due to the fact that scarcer and more supermassive stars may be higher in phosphorus than more common stars. Results from this research contradict the predictions made by computer models. According to the models, both the Cass A and the Crab nebulas should have contained a similar amount of phosphorus.
This discovery means that the emergence of alien life could rely, in large part, on mere luck. Not only must the planet be at the right location in its system and at the right location in its galaxy, but it must also trace its beginnings back to just the right sort of star.

So what happens when one of the elements essential to the emergence of life is a rare creation in our universe? Could other lifeforms have found a substitution for phosphorus in their DNA? When scientists this past year thought they’d discovered significant phosphorus spikes on Venus, the possibility of alien life surged. These spikes came in the form of phosphine molecules which contained atoms of hydrogen and phosphorus. Astronomers see phosphorus as a biosignature — evidence for the presence of life. Reexamination later revealed that these spikes were not as significant as researchers originally thought, greatly reducing the hypothesis of Venus being host to chemical-producing organisms.
Scarcity of this element works in favor of the Rare Earth hypothesis, a belief that there is no abundance of alien life in the universe because too many factors have contributed to the emergence of organisms here on Earth. The conditions which gave rise to us were more than just fortunate or difficult to replicate; if the Rare Earth hypothesis is correct then they were nearly miraculous. The rarity of phosphorus is coded into our very cells. A treasure of an element lives in